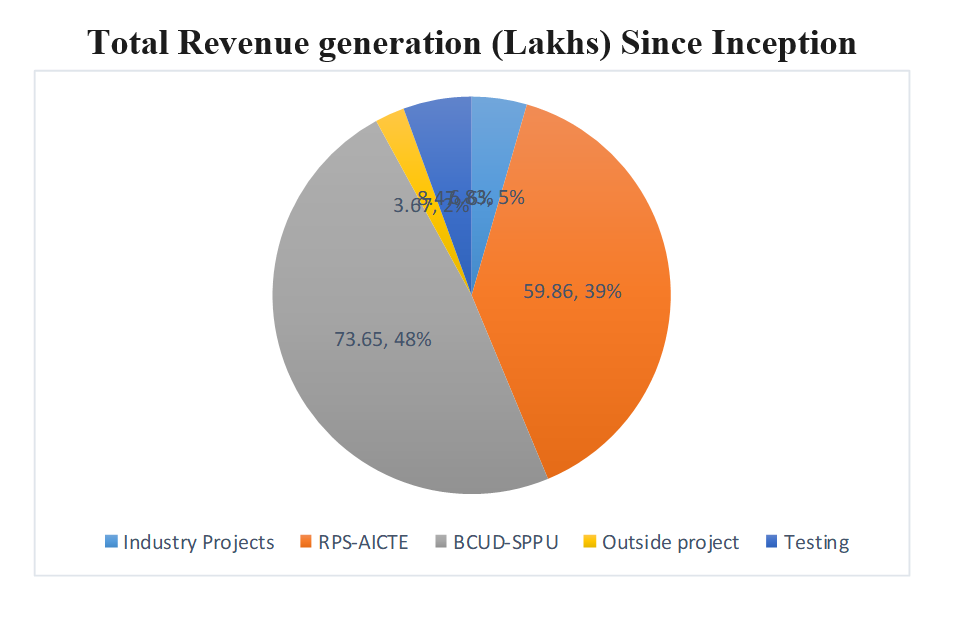
Overall Summary of Revenue (in Lakhs) from Projects and Testing (Last 5 Years)
| 2024-25 | 2023-24 | 2022-23 | 2021-22 | 2020-21 | Total Funding |
|---|---|---|---|---|---|
| 3.13 Lakhs | 4.03 Lakhs | 1.52 Lakhs | 1.10 Lakhs | 13.32 Lakhs | 23.1 Lakh |
Research Project Grants Summary
| Name of Principal Investigator | Grant Provided Under | Sanctioned Year | Status | Grant |
|---|---|---|---|---|
| Dr. A. R. Madgulkar | Major Research Project under Research Promotion Scheme, AICTE, New Delhi | 2013-14 to 2015-16 | Completed | 23.00 lakhs |
| Dr. M. R. Bhalekar | Major Research Project under Research Promotion Scheme, AICTE, New Delhi | 2017-18 to 2019-20 | Completed | 23.53 lakhs |
| Dr. S.V. Bhandari | Major Research Project under Research Promotion Scheme, AICTE, New Delhi | 2020-21 to 2022-23 | Completed | 13.33 lakhs |
| Grand Total = | 59.86 lakhs | |||
Savitribai Phule Pune University (Formerly University of Pune) Sponsored Minor Research Project Grants
| Sr. No. | Name of Principal Investigator | Title of Project | Sanctioned Year Period | Status | Amount Sanctioned |
|---|---|---|---|---|---|
| 1. | Dr. A. R. Madgulkar | Skin targeting using lipid Nanoparticles: Formulation and Evaluation | 2008-10 |
Completed
|
2,00,000/- |
| 2. | Dr. M. C. Damle | Simultaneous Determination of Drugs In Combination Dosage Forms by Stability-Indicating Method. | 2008-10 |
Completed
|
1,50,000/- |
| 3. | Dr S. V. Bhandari | Design, synthesis and biological evaluation of novel antineoplastic agents using molecular modeling studies | 2008-10 |
Completed
|
1,50,000/- |
| 4. | Mrs. M.R.P. Rao | Characterization and Evaluation of Natural Polysaccharide as Novel Pharmaceutical Excipient | 2008-10 |
Completed
|
2,00,000/- |
| 5. | Dr N. S. Vyawahare | Preparation and preclinical evaluation of herbal formulation for obesity | 2008-10 |
Completed
|
2,00,000/- |
| 6. | Dr. S. J. Kshirsagar | Design, Development of Oral Dosage Forms for Colon- Specific Drug Delivery Using Enteric Polymers | 2008-10 |
Completed
|
1,50,000/- |
| 7. | Dr. T. S. Chitre | Design, Synthesis And Biological Evaluation Of Some Non-Nucleoside HIV-1 RT Inhibitors Using Structure Optimization Through Molecular Modeling Studies | 2009-11 | Completed | 3,00,000/- |
| 8. | Mr. V. S. Joshi | Pharmacognostic and Pharmacological Evaluation of Immunomodulatory Activity of Some Traditional Medicinal Plants | 2009-11 | Completed | 2,00,000/- |
| 9. | Dr. S. V. Gandhi | Bioanalytical HPLC Method Development and validation for the estimation of lornoxicam | 2009-11 | Completed | 3,00,000/- |
| 10. | Dr. M. R. Bhalekar | Study of novel methods to improve the bioavailability of a drug by transdermal drug delivery system. | 2009-11 | Completed | 2,00,000/ |
| 11. | Mrs. M. V. Dhoka | Profile of the drugs used in combination for the treatment of Chronic bronchitis | 2009-11 | Completed | 2,00,000/ |
| 12. | Mrs. R. N. Mirajkar | Topical herbal Emulgel | 2010-12 | Completed | 2,00,000/ |
| 13. | Dr. M. M. Bandivadekar | Formulation and evaluation of solid self-emulsifying drug delivery system to improve the dissolution profile of poorly soluble drug | 2010-12 | Completed | 2,00,000/ |
| 14. | Mr. P. B Deshpande | Validated stability indicating method development for determination of drugs in pharmaceutical formulations. | 2010-12 | Completed | 1,50,000/- |
| 15. | Dr. A. R. Madgulkar | Formulation development and in-vivo evaluation of trans mucosal bioadhesive drug delivery system. | 2012-14 | Completed | 3,00,000/- |
| 16. | Dr. S. V. Bhandari | Development of selective anticancer agents by pharmacophore optimization using molecular modeling studies and synthesis, characterization, and in vitro screening using cell line assays and toxicity studies. | 2012-14 | Completed | 2,95000/- |
| 17. | Mr. R. R. Padalkar | Formulation development and in-vivo evaluation of nasal drug delivery system. | 2012-14 | Completed | 3,00,000/- |
| 18. | Mrs. A. A. Avalaskar | Formulation and evaluation of polyherbal tablets having Sesbania grandiflora as a major component for its anti inflammatory activity | 2012-14 | Completed | 2,20,000/- |
| 19. | Mrs. S. N. Narkhede | Preclinical evaluation of polyherbal formulation for female sexual dysfunction | 2012-14 | Completed | 2,00,000/- |
| 20. | Dr. Mangesh R Bhalekar | Development of chloroquine nanoparticles for oral treatment of Rheumatoid arthritis | 2013-15 | Completed | 2,50,000/- |
| 21. | Dr. Mrinalini C. Damle | Development and validation of stability indicating method for Active Pharmaceutical Ingredients. | 2013-15 | Completed | 1,50,000/ |
| 22. | Dr. Sanjay J. Kshirsagar | Design and development of Liposomes for colon targeted drug delivery | 2013-15 | Completed | 2,30,000/ |
| 23. | Dr. Trupti S Chitre | Development of Metallocenes: A modified Approach towards Resistant Tuberculosis | 2013-15 | Completed | 2,80,000/ |
| 24. | Dr. Ashwini R. Madgulkar | Permeation Enhancement of Poorly Bioavailable Drug Using Nanoparticulates Of Thiolated Xyloglucans | 2014-16 | Completed | 2,30,000/- |
| 25. | Dr. Monica R. P. Rao | Characterization and evaluation of modified polysaccharide as a novel pharmaceutical excipient | 2014-16 | Completed | 2,20,000/- |
| 26. | Dr. Shashikant V. Bhandari | Development of Potential Antitubercular agents using Pharmacophore optimization by Molecular Modelling Studies | 2014-16 | Completed | 2,20,000/- |
| 27. | Dr. Santosh V. Gandhi | Bioanalytical Method Development and Validation for Determination of Pharmaceuticals in Biological Samples | 2014-16 | Completed | 1,40,000/- |
| 28. | Mr. Padmanabh B. Deshpande | Stability indicating method development and validation for simultaneous estimation of drugs as bulk and in pharmaceutical formulations | 2014-16 | Completed | 1,00,000/- |
| 29. | Mrs. Shital M. Patil | Development of some Anti-HIV agents | 2014-16 | Completed | 1,60,000/- |
| 30. | Dr. M. C. Damle | Development and validation of stability indicating method for marker compound in selected herbs | 2016-18 | Completed | 1,00,000/- |
| 31. | Dr. Trupti Chitre | Metallocenes and their B-cyclodextrin conjugates: A step towards Resistant tuberculosis | 2016-18 | Completed | 1,90,000/- |
| 32. | Mrs. Kalyani Asgaonkar | Development of some Anti- tubercular agents | 2016-18 | Completed | 1,70,000/- |
| 33. | Mrs. Amruta Avalaskar | Formulation and standardization of Adhulsa lozenges for pediatric patients in the management of asthma | 2016-18 | Completed | 2,00,000/- |
| 34. | Mrs. Swati Narkhede | A study of nootropic activity of polyherbal formulation in laboratory animals | 2016-18 | Completed | 1,70,000/- |
| 35. | Mrs. Reshma Mirajkar | Formulation development of an in situ gel forming system for controlled delivery | 2016-18 | Completed | 1,70,000/- |
| 36. | Dr. Shital M. Patil | Development of some heterocyclic derivatives as EGFR inhibitors using pharmacophore optimization. | 2023-2024 | Ongoing | 2,75,000/- |
| TOTAL (Rs.) | 73,70,000/- | ||||